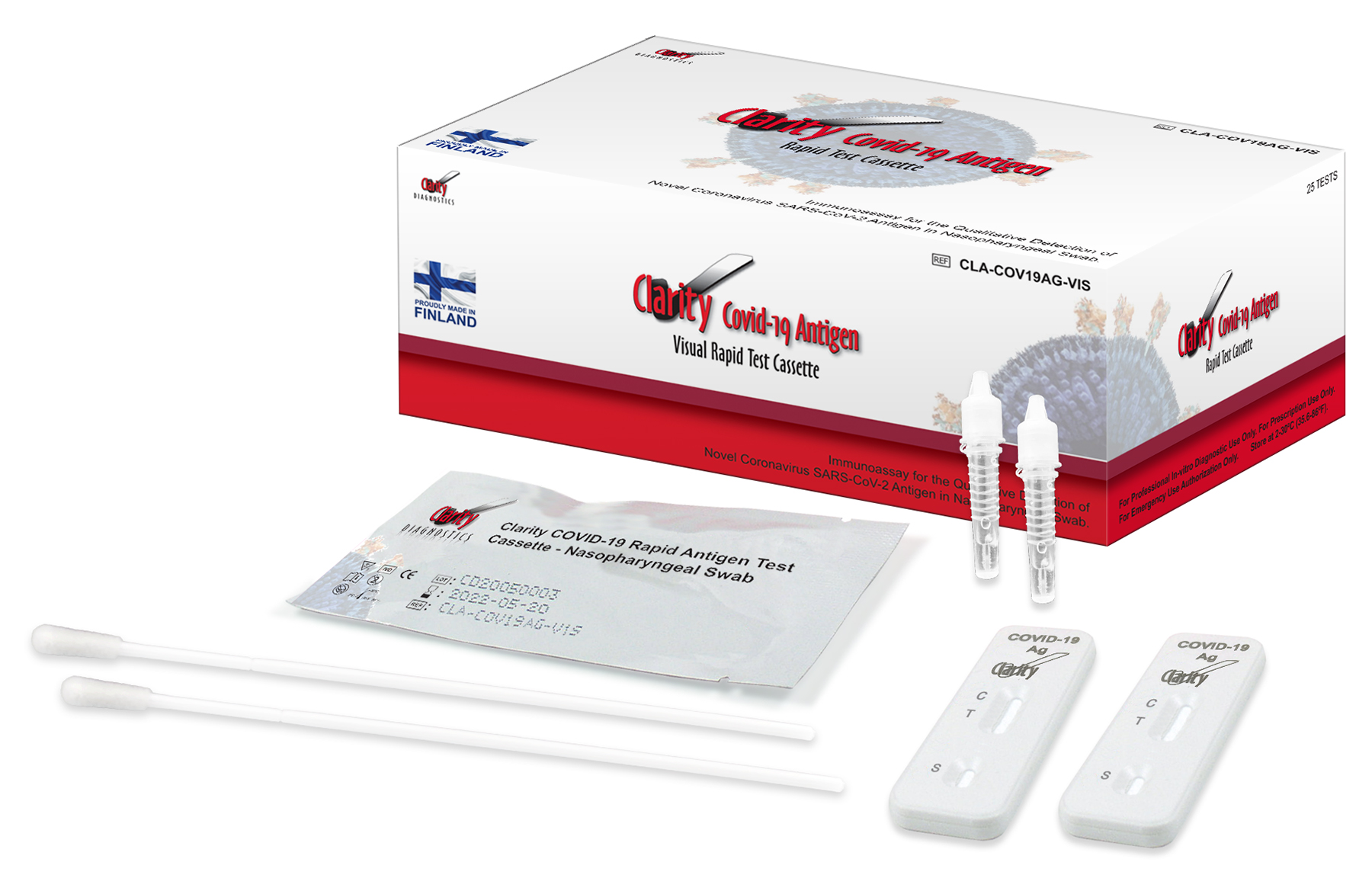
THE CLARITY COVID-19 ANTIGEN RAPID TEST CASSETTE IS NOW EUA, CLIA WAIVED AND POCT APPROVED
Clarity COVID-19 Antigen Rapid Test Kits, Includes:
(25) Tests
(25) NP Swabs
(25) Buffers
Package Insert
QSG
(1) Negative Control
(1) Positive Control/kt
Product Inserts Include:
- Clarity CLA-COV19AG-VIS Package Insert
- Fact Sheet for Healthcare Providers
- Fact Sheet for Recipients CLARITY COVID-19 ANTIGEN RAPID TEST CASSETTES
Benefits:
- FDA Emergency Use Authorized (EUA), CLIA Waived and POCT approved.
- Relative Sensitivity 87.5% - Relative Specificity: >98.9%
- Fast and Easy
- Positive results as fast as 5 minutes
- Test Naso-Pharyngeal Swab immediately, or store at room temperature and test within 1 hour.
- Naso-Pharyngeal Swab stored in a dry tube at 2-8c can be tested within 24 hours.
- EUA #: EUA210062
Product Codes CLA-COV19AG-VIS (25/box) Contains:
25 Individually pouched test cassettes, 25 Sterile Naso-Pharyngeal Swabs, 25 Buffer Tubes, 1 Negative Control Swab, 1 Positive Control Swab, 1 Work Station, 1 Package Insert, 1 Quick Start Guide.
IMPORTANT INFORMATION The Clarity COVID-19 Antigen test can be used to test directly collected naso-pharyngeal swab specimens.
The Clarity COVID-19 Antigen test should be ordered for the detection of COVID-19 antigen in individuals who are suspected of COVID-19 by their healthcare provider and who are within six days of symptom onset.
The Clarity COVID-19 Antigen test is authorized for use in laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. 263a, that meet requirements to perform high complexity, moderate complexity, or waived tests.
This test is authorized for use at the Point of Care (POC), i.e., patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
Our CLIA Waiver can be found on the FDA's website at the following link: https://www.fda.gov/media/149056/download
Covid Attributes: Type: Antigen / Rapid
Use: Lab / Clinical
Certification: CLIA Certificate Required for purchase
Sample SpecimenType: Nasal Swab
Availability: Ready to ship (typically ships within 7 days)
Product Video
The Clarity COVID-19 Antigen test is authorized for use in laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. 263a, that meet requirements to perform high complexity, moderate complexity, or waived tests. This test is authorized for use at the Point of Care (POC), i.e., patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
- Requires a CLIA Certificate Number for purchase
- Orders are Non-Cancellable; Item is Non-Returnable

MedPlus:
MedPlus Services USA, exclusively for medical and dental distributors, is your one-stop, single source for purchasing medical and dental products and supplies at competitive prices. With over 300 healthcare and dental manufacturers' products in stock and available from our warehouses, you are well positioned to meet the needs of your customer. More importantly, you will be competitive in today's challenging marketplace. MedPlus Services allows you to bundle your product purchases from manufacturers like: 3M, BD, Covidien/Kendall, Alere, Smith & Nephew and over 300 more onto one single purchase order.
- All items are not authorized for resale.